Wednesday, October 29, 2003
3053
P31: Breast Augmentation: Thirty Nine Years after Foam Implantation - A Case Report
The attraction of larger breasts has always seemed to be more important than the risks associated with implantation. We would like to present a case report that may be the longest lasting case of foam breast implantation.
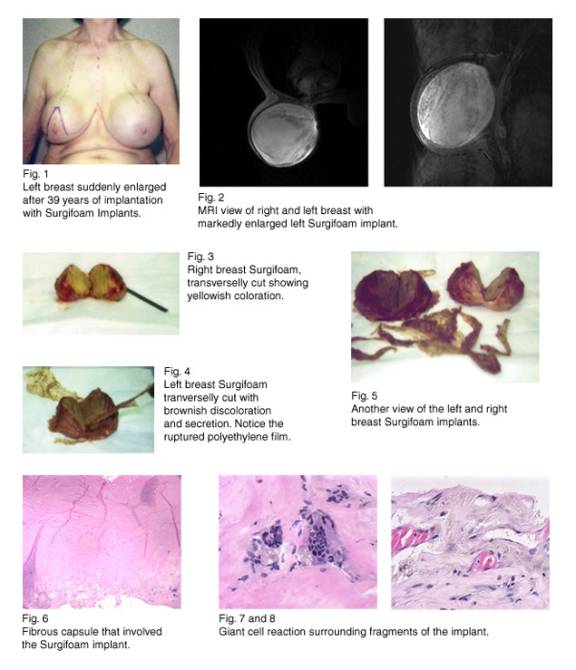
A 73 year-old woman, 39 years ago, after reading a book on a novelty technique on breast augmentation, traveled from Wyoming to Los Angeles and underwent the procedure without inquires or explanation. She has been satisfied with two hard round breasts. Recently, she developed abrupt significant enlargement of her left breast without other symptoms. There was no history of trauma.
With a possible diagnosis of left breast tumor, a mammogram was requested, which demonstrated bilateral breast implants of different size with unknown substance. No malignancy was identified. The patient desired removal of both implants immediately.
At surgery, Franklyn Surgifoam breast implants were removed. The right implant was of dark-yellowish color surrounded by pieces of celluloid-like ragged material, contained in an intact thick fibrous calcified capsule. On the left, the implant was almost doubled in size, soaked in a dark-brown liquid with similar pieces of celluloid-like wrapping and a burst fibrous capsule. The fibrous capsule showed a foreign body reaction without malignancy, and the cultures were negative.
The Franklyns Surgifoam implants were made of polyester foam wrapped in a polyethylene film jacket, either with a knotted or heat-sealed closure. The outer film membrane was supposed to prevent the ingress of tissue. The devices were made exclusively for Dr. Robert Franklyn of L.A. from 1958-1965. During World War II, while visiting Canada, he noted a seat cushion in a captured German aircraft made of a unique type of a synthetic foam material, which he thought would be suitable for surgical implantation. After the war, he developed breast implants with this material under the name of "Surgifoam." This product was marketed before they could be tested in animals. The main problems with the Franklyn prosthesis include degradation, resorption, and disintegration of the foam; perforation of the bag leading to flooding and swelling of the prosthetic compartment; and gross contamination. These adverse effects lead to explantation and debridement.
View Synopsis (.doc format, 26.0 kb)
See more of Posters
Back to Plastic Surgery 2003 Complete Scientific Program
Back to Plastic Surgery 2003 Meeting home